What is a metal?

Humans have been using metals for over 7000 years and they have a wide variety of applications. They are one of our most important resources.
Iron, magnesium and gold are examples of metal elements.

Physical properties of metals
Metals have a number of physical properties in common:
They are:
- shiny, especially when they are freshly cut (we say that they are lustrous)
- good conductors of electricity
- good conductors of heat
- malleable which means they can be bent and shaped without breaking
- ductile which means they can be drawn out into a tiny wire

Most metals also have other physical properties in common. They are:
- solid at room temperature, except mercury
- high melting point
- hard
- strong
- they have a high density
- they are sonorous, which means they make a ringing sound when hit тАУ thatтАЩs why bells are made from metal

Three metals (iron, cobalt and nickel) are magnetic. Steel is an alloy, a mixture of iron and carbon and other metals (see below), but it is mostly iron, and it is also magnetic. The other metal elements are not magnetic.
Approximately 75% of elements in the periodic table are metals. They occupy the region from the staircase line across the middle of the table and over to the left-hand side.
Uses of metals
The oldest known metal object ever found is a copper awl, discovered at the Tel Tsaf excavation site near Israel's border with Jordan. It dates back to the late 6th or early 5th century B.C. The awl тАФ a sharp object usually meant for poking small holes in leather or wood тАФ measures only 4 cm long and is set in a wooden handle. Ever since that time humans have come to understand and appreciate the various physical properties of metals and have developed a large number of uses for them.
| Metal or alloy | Physical property | Objects made from the metal |
|---|---|---|
| Copper | Good conductor of electricity, Ductile | Used in electric cables and wires |
| Iron and steel | Hard, strong | Bridges, ships, cars, girders for buildings |
| Gold, platinum and silver | Shiny, ductile, malleable, unreactive | jewelleries and ornaments, coins |
| Aluminium | Good conductor of heat, malleable, low density | Pots and pans, aluminium foil, aircraft parts |
| Brass | Malleable, ductile, shiny | Musical instruments, door knobs, locks, taps |
| Bronze | Hard, sonorous, ductile, strong | Bells, coins, statues |
| Mercury | Liquid at room temperature, good conductor of heat, shiny | Thermometers, barometers, Mercury switches |
Alloys
An alloy is a mixture of two or more elements, where at least one element is a metal. Many alloys are mixtures of two or more metals. Most alloys are made by melting the metals, mixing them while they are liquid to form a solution, then leaving them to cool and turn solid again.
Normally alloys are made to improve the properties of the metals used in the mixture, especially to make them harder, give greater strength and resistance to rust and wear.
Common alloys include:

- Steel тАУ an alloy of iron, carbon and other metals. Stronger than pure iron
- Brass тАУ an alloy of copper and zinc. Stronger and stiffer than copper
- Bronze тАУ an alloy of copper, tin, other metals and sometimes non-metals. Harder than copper and more resistant to corrosion
- Solder тАУ an alloy of tin and lead. Lower melting point than both metals
- Neodymium alloy тАУ an alloy of neodymium, iron and boron. Makes much stronger magnets than iron which hold their magnetism for longer
- Stainless Steel - an alloy of chromium and nickel. Hard and resistant to rusting

Chemical properties of metals
We can examine the reactivity of metals by observing their reactions with oxygen, water, steam and dilute acids.
Reaction of metals with oxygen - oxidation
Metals react with oxygen in air to produce compounds called metal oxides.
metal + oxygen тЖТ metal oxide
Observations and equations for reaction of some metals in the reactive series with air, are shown in the table below.
| Metal | Word Equation | Symbol Equation | Observations |
|---|---|---|---|
| potassium | potassium + oxygen тЖТ sodium | 4K(s) + \(O_2\) тЖТ 2\(K_2\)O(s) | burns with a lilac flame producing a white solid |
| sodium | sodium + oxygen тЖТ sodium oxide | 4Na(s) + \(O_2\) тЖТ 2\(Na_2\)O(s) | burns with a yellow flame producing a white solid |
| calcium | calcium + oxygen тЖТ calcium oxide | 2Ca(s) + \(O_2\)(g) тЖТ 2CaO(s) | burns with a red flame producing a white solid |
| magnesium | magnesium + oxygen тЖТ magnesium oxide | 2Mg(s) + \(O_2\)(g) тЖТ 2MgO(s) | burns with white light producing a white solid |
| aluminium | aluminium + oxygen тЖТ aluminium oxide | 4Al(s) + \(3O_2\)(g) тЖТ \(2Al_2\)\(O_3\)(s) | burns only when finely powdered producing a white solid |
| zinc | zinc + oxygen тЖТ zinc oxide | 2Zn(s) + \(O_2\)(g) тЖТ 2ZnO(s) | burns producing a yellow solid which becomes white on cooling |
| iron | iron + oxygen тЖТ iron oxide | 3Fe(s) + \(2O_2\)(g) тЖТ \(Fe_3\)\(O_4\)(s) | iron filings burn with orange spark producing a black solid |
| copper | copper + oxygen тЖТ copper(II) oxide | 2Cu(s) + \(O_2\)(g) тЖТ 2CuO(s) | red brown copper glows red, there is a blue-green flame and it becomes covered with a black layer |
The more reactive metals burn completely when heated. However, less reactive metals such as copper only react on the surface and a layer of product forms.

A reaction in which a substance combines with oxygen in this way is an example of oxidation.For example, magnesium burns in air easily with a white light, to produce magnesium oxide. The reaction can be represented by this word equation:
magnesium + oxygen тЖТ magnesium oxide

Most metals are shiny when freshly polished or cut but become dull and tarnished over time. This occurs because of a slow chemical reaction between the metal surface and the oxygen in air. The reaction produces a coating of metal oxide on the surface of the metal.
copper + oxygen тЖТ copper oxide
This type of chemical reaction is called corrosion. The compound, copper oxide, is formed on the surface of the metal.
Copper oxide is black and in time will coat the surface of copper left in air. Heating the copper speeds up the reaction. If a piece of copper is heated it quickly becomes coated in black copper oxide.



Lead normally appears to have a dull surface. This is due to corrosion, with a coating of lead oxide formed on the lead surface, as the lead reacts slowly with the oxygen in the air.
lead + oxygen тЖТ lead oxide
The lead is protected from further corrosion by the layer of lead oxide on the surface. Polishing or scratching the lead reveals shiny lead beneath the surface.

Similar corrosion reactions occur with other metals such as magnesium and aluminium.
Question
Within a reaction what solid and gas are needed to make magnesium oxide?
Magnesium and oxygen have to react together to make magnesium oxide.
magnesium + oxygen тЖТ magnesium oxide
Question
What is made when aluminium and oxygen react together?
When aluminium and oxygen react together you get aluminium oxide.
aluminium + oxygen тЖТ aluminium oxide.

Potassium and sodium are soft metals which are easily cut, exposing a shiny surface which changes to dull rapidly. The change from shiny to dull is called tarnishing.
For example, potassium burns with a lilac flame producing a yellow, grainy solid when heated in air.
potassium + oxygen тЮЮ potassium oxide
Sodium burns with a yellow flame producing a yellowish solid
sodium + oxygen тЮЮ sodium oxide
Metal oxides are bases. They react with acids and neutralise them. Some metal oxides dissolve in water and, when they do, they produce alkaline solutions.

Reaction of metals with water
Metals react with water in a variety of ways. Some react slowly, some react readily and violently.
When a metal reacts with water, a metal hydroxide and hydrogen are formed.
metal + water тЮЮ metal hydroxide + hydrogen
Potassium reacts violently with water. It bursts into lilac-coloured flames because so much heat energy is given out. The flame is the result of the hydrogen gas burning. The potassium shoots rapidly across the surface of the water, propelled by the hydrogen gas.
The word equation is:
potassium + water тЮЮ potassium hydroxide + hydrogen
Sodium reacts vigorously with water.
sodium + water тЮЮ sodium hydroxide + hydrogen
The reaction of sodium and water produces a lot of heat energy. The metal sodium melts and round balls of liquid sodium fizz across the surface of the water. They are moved about by the hydrogen gas produced.
These are examples of exothermic reactions тАУ heat energy is given out.
Potassium and sodium are so reactive that they are stored under oil. This prevents oxygen and water reaching them.
Calcium reacts steadily with water.
calcium + water тЮЮ calcium hydroxide + hydrogen
The apparatus below is used to react calcium with water and collect the gas. This would not be used with sodium or potassium as they float, and the reaction is too vigorous.
Bubbles of gas are released, and heat energy is given out. Calcium hydroxide is only slightly soluble in water, so the solution quickly becomes milky as solid calcium hydroxide appears.
The gas collected in the boiling tube can be tested for hydrogen - a lighted wooden splint makes a popping sound in a boiling tube of hydrogen.
Calcium is stored in a closed bottle but not under oil because it reacts with water less violently than potassium or sodium.
Reactions of metals with dilute acid
Most metals react with dilute acids and when they do, a salt of the metal and hydrogen gas are formed. The salt is named following the rules you learned in the Acid and alkalis study guide.
acid + metal тЖТ salt + hydrogen
For example
magnesium + hydrochloric acid тЖТ magnesium chloride + hydrogen
Using a metal and an acid to produce hydrogen and a salt
Watch the video below to see the correct procedure for reacting zinc and sulfuric acid. This produces hydrogen and zinc sulfate, both of which can be tested for, but donтАЩt try to recreate these experiments at home as they may cause injury
Reactions of metals with steam
Magnesium reacts very slowly with water. However, it reacts vigorously with steam:
magnesium + steam тЖТ magnesium oxide + hydrogen
Metals which react with steam form the solid metal oxide and hydrogen gas.
In general, the more reactive the metal, the more rapid the reaction.
Aluminium is unusual, because it is a reactive metal that does not react with water. Its surface forms a protective layer of aluminium oxide that keeps water away from the metal below.
The apparatus used to react a metal with steam and collect the gas produced is shown below.
The damp mineral wool is heated to generate steam.
Making Hydrogen.
DonтАЩt try to recreate these experiments at home as they may cause injury.
Question
Can you write the word equation for this reaction?
zinc + sulfuric acid тЖТ zinc sulfate + hydrogen
Metals such as lead, tin, magnesium and iron react with dilute acids. Some metals, like copper, gold and silver do not react with most dilute acids.
Potassium and sodium are much too dangerous to react with dilute acid! The reaction is dangerously fast and potentially explosive.
Summary
| Metal | Reaction with oxygen when heated | Reaction in cold water | Reaction in dilute acid |
|---|---|---|---|
| potassium | Reacts vigorously | Reacts vigorously | Reaction too dangerous to be attempted. |
| sodium | Reacts vigorously | Reacts vigorously | Reaction too dangerous to be attempted. |
| calcium | Reacts vigorously | Reacts vigorously | Reaction too dangerous to be attempted. |
| magnesium | Reacts readily | Reacts slowly | Fast reaction |
| aluminium | Reacts readily | No reaction | Fairly fast reaction |
| zinc | Reacts steadily | No reaction | Fairly fast reaction |
| iron | Reacts steadily | No reaction | Fairly fast reaction |
| tin | At room temperature slowly forms oxide that coats surface | No reaction | Reacts slowly |
| lead | At room temperature slowly forms oxide that coats surface | No reaction | Reacts slowly |
| copper | At room temperature slowly forms oxide that coats surface | No reaction | No reaction with most dilute acids |
The most reactive metal is at the top of the table and the reaction level gets less as you go down the table. It is clear from this table that there is a pattern that enables us to put the metals in order of how quickly they react and their readiness to take part in chemical reactions.
The reactivity series of metals is a chart listing metals in order of decreasing reactivity. In general, the more reactive a metal is:
- the more vigorously it reacts with other substances
If we put the metals in order of their reactivity, from the most reactive down to the least reactive, we get the reactivity series.
If you want to learn the reactivity series, you could try making up a mnemonic or silly sentence to help. Here's one:
"Please Send Charlie's Monkeys And Zebras In Tough Large Cages Securely Guarded Properly."
Displacement reactions
Displacement reactions in metals
Displacement reactions involve a metal and a compound of a different metal.
In a displacement reaction:
- a more reactive metal will displace a less reactive metal from its compounds
Displacement reactions are easily seen when a salt of the less reactive metal is in the solution.
During the reaction:
- the more reactive metal gradually disappears as it forms a metal (salt) solution
- the less reactive metal coats the surface of the more reactive metal
For example, magnesium is more reactive than copper. When a piece of magnesium is dipped into blue copper sulfate solution:
- the blue colour fades as colourless magnesium sulfate solution forms
- red-brown copper coats the surface of the magnesium
The copper is pushed out or displaced from the solution of copper sulfate by the more reactive magnesium, which replaces it.Here is the word equation for the reaction:
magnesium + copper (II) sulfate тЖТ magnesium sulfate + copper
No reaction is seen if you do things the other way round тАУ in other words, if you put copper into magnesium sulfate solution. This is because copper is not reactive enough to displace magnesium from magnesium sulfate.
The metal that does the replacing is always more reactive than the replaced metal. Magnesium replaces copper - magnesium is more reactive than copper.
Investigating displacement
You can investigate the reactivity of metals using displacement reactions. The table shows the results from a series of experiments involving four metals and solutions of their salts. A tick shows where there is a visible reaction and a cross shows where there is no visible reaction.
| Magnesium | Zinc | Iron | Copper | |
|---|---|---|---|---|
| Magnesium sulfate | x | x | x | x |
| Zinc sulfate | тИЪ | x | x | x |
| Iron sulfate | тИЪ | тИЪ | x | x |
| Copper sulfate | тИЪ | тИЪ | тИЪ | x |
| Reactions seen | 3 | 2 | 1 | 0 |
Magnesium displaces three metals, zinc displaces two metals, iron displaces one metal and copper does not displace any of the other three metals. A reaction only happens when the metal added is higher in the Reactivity Series than the metal in the saltSo, the order of reactivity, starting with the most reactive first, is:
- magnesium
- zinc
- iron
- copper
Displacement reactions can also involve solid metal oxides.For example:
Zinc + copper oxide тЖТ copper + zinc oxide
Zinc displaces the copper which occurs because zinc is more reactive than copper. Copper will not displace zinc from zinc oxide as copper is less reactive than zinc.
Why displacement reactions are important
When looking at reactions of metals with oxygen, water and dilute acid, it is sometimes difficult to compare their reactivity. For instance, when observing tin and lead reacting with dilute acid тАУ they both produce bubbles slowly but is it the tin or lead which is the more reactive?
Using displacement reactions is much easier, and more accurate way of producing a Reactivity Series. The more reactive metal always displaces the less reactive metal in a compound and never the other way about, so there is no margin for error.
Chemists can then use the Reactivity Series to make confident predictions about how metals will react and how they can be extracted.
Obtaining metals from metal oxides
Carbon is a non-metal, but it is more reactive than some metals. This means that some metals can be extracted from their metal oxides using carbon.
Less reactive metals than carbon can be extracted from their oxides by heating with carbon. In general:
metal oxide + carbon тЖТ metal + carbon dioxide
This works for zinc, iron, tin, lead and copper as they are less reactive than carbon. Copper is the least reactive of these five metals.
To extract copper, you mix copper oxide powder with carbon powder. You then heat the mixture strongly for a few minutes in a crucible. It is important to keep a lid on the crucible, otherwise the carbon will react with oxygen in the air, rather than with the copper oxide. The carbon dioxide formed in the reaction escapes into the air.
After letting the crucible cool down, you tip the mixture into cold water. Pieces of brown copper sink to the bottom, leaving unreacted powder suspended in the water.
The word equation for the reaction is:
copper oxide + carbon тЖТ copper + carbon dioxide

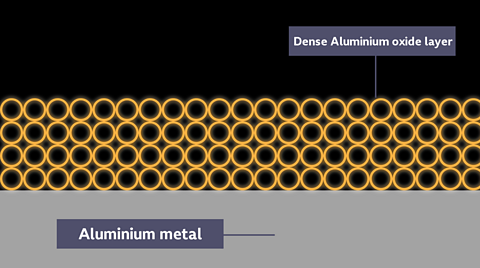
Note that aluminium can be difficult to place in the correct position in the reactivity series during these experiments. This is because its protective aluminium oxide layer makes it appear to be less reactive than it really is.

Extracting iron from iron oxide
Iron is less reactive than carbon, so it can be extracted from iron oxide using carbon. This is done on an industrial scale in a huge container called a blast furnace.
Lumps of iron oxide are mixed with carbon and dropped into the top of the blast furnace. Hot air is blasted in at the bottom. The oxygen in the air reacts with the carbon, forming carbon monoxide:
carbon + oxygen тЖТ carbon monoxide
It is hot enough in the blast furnace for the carbon monoxide to react with the iron oxide:
iron oxide + carbon monoxide тЖТ iron + carbon dioxide
The carbon displaces the iron leaving molten iron and carbon dioxide gas. The molten iron flows to the bottom of the blast furnace, where it runs into moulds, cools and solidifies. This is called cast iron which is very brittle. It is normally processed further before it can be used.

Corrosion and rusting
As we saw earlier in this study guide corrosion occurs when a metal reacts with oxygen in air to form a coating of its oxide. It is usually a slow reaction and metals like gold and platinum donтАЩt corrode in air.
If a metal continues to oxidise in this way, it can become weaker over time and eventually all of it may become metal oxide.

Rusting is the special name for the corrosion of iron and steel. It occurs when iron or steel reacts with oxygen in air and water. The chemical name for rust is hydrated iron (III) oxide and it is a flaky brown solid.
Rusting experiments
Experiment 1
What conditions are needed for rusting to occur?
The experiment in the diagram shows that both oxygen and water are needed for rusting to happen.
Three identical nails were placed in test tubes as shown below with the contents of each test tube as indicated. The test tubes were left at room temperature in a test tube rack for five days. They were then carefully examined for rust. The results were recorded in a suitable table.
Results
After five days:
| Test tube | Conditions | Outcome |
|---|---|---|
| left-hand test tube | air and water | nail rusted |
| middle test tube | water but no air | nail did not rust |
| right-hand test tube | air but no water | nail did not rust |
ConclusionAir and water are both needed for rusting to occur.
The experiment can be repeated with the left-hand test tube containing air and water, the middle test tube containing salt dissolved in water (like sea water) and the right-hand test tube containing dilute acid. After five days it is found that the nail in water is rusty, and both other nails are very rusty.
This shows that if water contains salt or acid the rusting reaction speeds up.
Experiment 2
Does the mass of iron change when it rusts?
Put dry iron filings into an evaporating dish.
Measure the mass of the iron filings and dish using a top pan balance. Record your answer.
Add water to the iron filings and leave the dish on a windowsill for a week. During this time the water will have evaporated, and the iron filings will have rusted.

Ensure the iron filings are dry and then measure the mass of rusted iron filings and evaporating dish. Record your results in the table.
Calculate any difference in mass.
Conclusion
An increase in mass occurs when iron rusts. Iron has combined with some part of the air or water.
Experiment 3
What does iron combine with when it rusts?
Place some iron filings in the bottom of a damp graduated cylinder which is exactly 100 cm3. The iron filings will stick to the side of the cylinder.
Carefully invert the cylinder in a beaker of water. Leave at room temperature for one week.
After one week the iron filings have rusted and water has risen into the cylinder.
Determine the volume of air left in the cylinder. Test the gas in the cylinder with a lighted splint
Conclusion
The results show that of the 100 cm3 of air present at the start, 21% is used up when iron rusts. This is the same as the percentage of oxygen in air. The lighted splint goes out showing that there is no oxygen remaining.
From these experiments we can conclude that:
- When iron rusts it takes on oxygen in air
iron + oxygen тЖТ iron oxide
Rust is more accurately hydrated iron oxide. Iron reacts with oxygen and water.
The word equation for rusting is:
iron + oxygen + water тЖТ hydrated iron oxide
The experiments also showed that salt water and dilute acid speed up rusting. So iron ships, bridges and buildings, in or near seawater, will rust rapidly and acid rain will speed up the rusting of iron railings, cars and bicycles.
Why rust is a problem?

Metals containing iron, such as most kinds of steel, will rust when exposed to air and water. Rusting is a problem because it effects iron and steel objects in a number of ways.
They will look orange and rough
It makes them weaker, by replacing the strong iron or steel with flaky, powdery iron oxide
Rust does not provide a protective coating for the metal surface preventing further rusting but slowly eats it away, greatly reducing its strength
Rust can cause a great deal of friction between metal parts that are supposed to slide over one another and they can become stuck together тАУ like a rusty door hinge, a rusty screw or a nut and bolt
Rust reduces ironтАЩs magnetic properties making for a much weaker magnet
Rust doesn't conduct electricity easily and so iron wires or contacts stop a circuit working if they rust.

Preventing rusting
There are several ways to prevent iron and steel rusting. Some work because they stop oxygen or water reaching the surface of the metal. This is called a physical barrier.
Examples of physical barriers include:
- oiling, for example, bicycle chains
- greasing, for example, nut and bolts
- painting, for example, car body panels
- coating, with a thin layer of plastic
- electroplating, coating with an expensive metal such as chromium, for example taps and cutlery
Question
Explain why a bike chain is protected from rusting by oiling it, rather than by painting it
As well as providing a physical barrier against rust, oil also lubricates the chain, reducing friction and helping it to move smoothly
Paint would just flake off when the bike is ridden, exposing the steel chain to air and water for it to rust again
Sacrificial protection
Iron can be protected from rusting if it is in contact with a more reactive metal, such as zinc or magnesium. The more reactive metal oxidises more readily than iron, so it тАШsacrificesтАЩ itself while the iron does not rust. Once the sacrificial metal has corroded away, it can simply be replaced. The Reactivity Series helps select a suitable metal to sacrifice.
Magnesium, aluminium and zinc are often used as sacrificial metals. They are more reactive than iron and prevent iron from becoming oxidised.
Examples of sacrificial protection
- hulls of ships тАУ zinc bolted to the shipтАЩs hull
- water heaters тАУ aluminium attached to the side of the heater
- underground oil pipes and tanks тАУ magnesium attached
- oil rigs тАУ magnesium attached to oil rigs at sea

The sacrificial metal must be periodically inspected and replaced because it is the metal that corrodes.
Question
Three nails are left in contact with air and water for a few days. A nail wrapped in magnesium does not rust. A nail alone rusts but a nail wrapped in copper rusts more. Explain these observations.
- magnesium is more reactive than iron - it oxidises more readily than iron so the nail does not rust
- iron is more reactive than copper - this means it oxidises more readily than copper, so it rusts faster than the nail alone
Galvanising

Galvanising is another method of rust prevention. An iron or steel object is coated in a thin layer of zinc. This stops oxygen and water reaching the metal underneath тАУ and the zinc also acts as a sacrificial metal.
Advantages of galvanising
- cheap
- low maintenance
- long lasting protective coat
- tough coating
- reliable means of protection

Examples of galvanising
- buckets
- galvanised iron nails
- nuts and bolts
- bicycles
- car bodywork
- bridge girders

Summary: Metals
metals are shiny, good conductors of heat and electricity, malleable and ductile
metal + oxygen тЖТ metal oxide
metal + water тЮЮ metal hydroxide + hydrogen
Test for hydrogen - a lighted wooden splint makes a popping sound in a boiling tube of hydrogen
- metal + acid тЖТ salt + hydrogen
Reactions of metals with steam
- metal + steam тЖТ metal oxide + hydrogen
Hydrochloric acid produces salts called metal chlorides
Sulfuric acid produces salts called metal sulfates
Nitric acid produces salts called metal nitrates
Displacement reaction for a metal - a more reactive metal will displace a less reactive metal from its compounds
A mnemonic for the reactivity series of metals - Penguins sure can make a zoo interesting - though lions catch some golden publicity
Rusting is the special name for the corrosion of iron and steel
Air and water are both needed for rusting to occur
- Galvanising - an iron or steel object is coated in a thin layer of zinc to protect from rust
Metals are never dull тАУ they just corrode a bitтАж

More on Chemistry
Find out more by working through a topic
- count7 of 8

- count8 of 8

- count1 of 8

- count2 of 8
