FDA panel backs first over-the-counter birth control pill in US
- Published
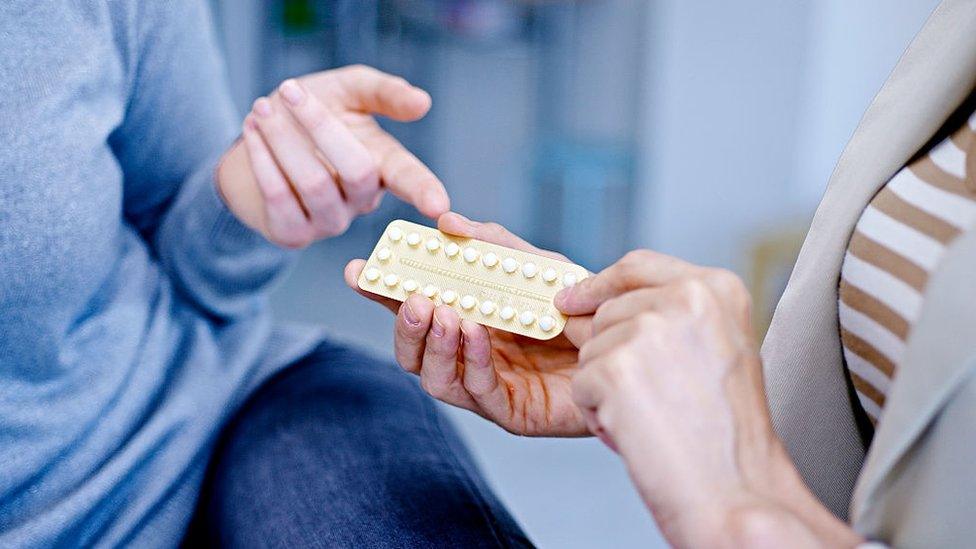
The first non-prescription birth control pill in the US is on the way to approval, after a thumbs-up from an advisory committee of drug regulators.
The Food and Drug Administration (FDA) panel's unanimous vote is not binding, but means the agency is likely to formally approve the drug this summer.
Opill has been available, but only by prescription, for the past 50 years.
Over-the-counter birth control is available in over 100 countries, says Free the Pill, an advocacy group.
The push for over-the-counter access in the US comes amid Republican-led efforts to restrict access to abortion and contraception at the national and state level.
Birth control pills are widely prescribed in the US, but nearly all US patients receive a pill that includes the oestrogen hormone.
Opill is a progestin-only pill, meaning that it is made of a synthetic form of progesterone and does not include oestrogen. It is taken once daily and must be consumed at the same time every day.
Seventeen outside experts from two FDA advisory panels met on Tuesday and Wednesday to deliberate over the safety and effectiveness of Opill.
In briefing documents published last week, FDA officials had expressed scepticism over an updated analysis of the pill provided by the drug maker.
They wrote that HRA Pharma appeared to have relied on low-quality studies, some dating back to when the drug was first approved in 1973.
Despite these reservations, the panel voted 17-0 on Wednesday in support of switching the pill from prescription to over-the-counter.
Advisors on the panel said they were mostly confident women of all ages would use the drug as appropriate without first consulting a healthcare provider.
"In the balance between benefit and risk, we'd have a hard time justifying not taking this action," said chairwoman Maria Coyle, an Ohio State University pharmacist.
"The drug is incredibly effective, and I think it will be effective in the over-the-counter realm just as it is in the prescription realm."
HRA's parent company Perrigo has told investors it expects the FDA to make a final decision within the next three months, the 成人快手's US partner CBS News reports.
If approved, Opill could be rolled out in pharmacies by the end of the year.